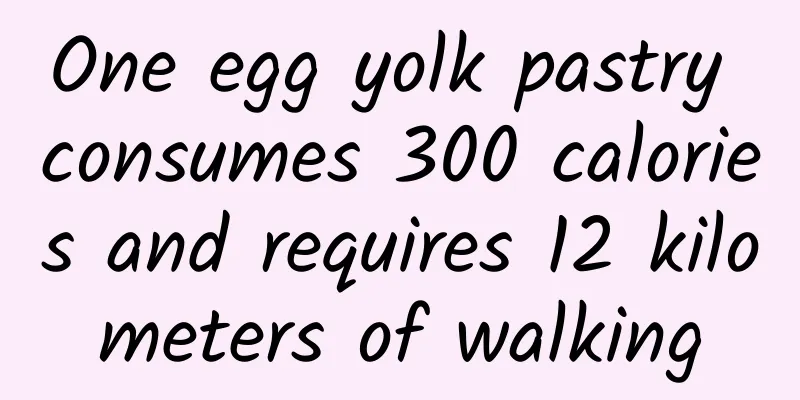Consumer Foundation: The ADI value of clenbuterol is still questionable

|
The Food and Drug Administration of the Department of Health, Executive Yuan, invited 16 experts and scholars to conduct an assessment of the acceptable daily intake (Acceptable Daily Intake, hereinafter referred to as ADI value) of ractopamine hydrochloride for humans, which will serve as the basis for the country's health risk assessment of ractopamine residues in food. The Consumers' Foundation held a meeting today, the 22nd, and stated that the participating committee members of the meeting were not representative enough and failed to include experts from all parties. Whether such deliberations were sufficient, and the content of the safety assessment may be questionable. Consumer Foundation Chairman Su Jinxia called on Legislative Yuan member Shen Yi to include ADI intake in risk assessment in the draft bill related to clenbuterol. (Photo provided by Consumer Foundation) The chairman of the Consumer Foundation, Su Jinxia, pointed out that the Executive Yuan made a surprise announcement at around 11 p.m. on March 5 to conditionally lift the ban on clenbuterol, and outlined a 16-word policy direction of "safety allowed, separation of cattle and pigs, mandatory labeling, and exclusion of offal." On April 11, the Food and Drug Administration of the Department of Health held a "Food Hygiene and Safety Risk Assessment Meeting" and proposed a resolution to establish an ADI (allowable daily intake per kilogram) assessment report, and recommended that the ADI value for the animal medicated feed additive ractopamine be 1 microgram/kg body weight/day. This ADI value of 1 microgram/kg body weight/day has taken into account the individual differences among human groups, including infants under 18 months old whose glucuronic acid metabolism function is not yet mature, children and adolescents over 18 months old, those with poor liver and kidney function and genetic polymorphism of beta-receptor, as well as sensitive groups such as patients with cardiovascular disease. Chairman Su Jinxia believes that the Food and Drug Administration of the Department of Health, Executive Yuan, invited 16 experts and scholars to conduct an assessment of the acceptable daily intake (ADI) value of ractopamine hydrochloride for humans, which will serve as the basis for our country's health risk assessment of ractopamine residues in food. However, the committee members participating in the meeting were not representative enough and experts from all sides were not included. Whether such deliberations are sufficient and the content of the safety assessment may be questionable. Even the format and invitees of the "Food Safety Briefing" currently being held nationwide by the Department of Health may have become an endorsement. We call on all legislators to review the Food and Health Act amendment bill next Monday and Wednesday, and not to put the health of the people at risk due to problematic procedures. Dean Chou Chin-cheng of the National Taiwan University College of Veterinary Medicine pointed out that there are no detailed reports and relevant supporting literature on the pharmacological effects, toxicological reactions, administration to animals (doses, reactions, and NOEL doses for monkeys and dogs, etc.), and countries that allow the use of clenbuterol. In the dose-effect assessment part, the Food and Drug Administration of the Department of Health did spend some time to understand a series of animal toxicology tests, including single-dose oral toxicity tests, in vitro and in vivo genotoxicity tests, multiple experimental animal species, repeated-dose toxicity tests for up to one year of daily oral administration, second-generation reproductive toxicity tests, and carcinogenicity research tests. However, there is still no relevant literature on special toxicology tests for cardiovascular reactions that the Chinese are most concerned about. Dr. Mackay-So also pointed out that food safety cannot be based on "acute toxicity" as the only risk assessment criterion. Chronic poisoning is a serious problem that requires more careful study. Moreover, organ toxicity studies of ractopamine should include cardiac muscle toxicity, skeletal muscle toxicity, teratogenicity, reproductive toxicity and genotoxicity, as well as psycho-behavioral toxicity. These related studies were not taken into consideration in this risk assessment, which obviously violated the risk assessment guidelines of international organizations. In addition, Dr. Weishuo emphasized that the assessment did not include an important risk group, the "fetus", which is also questionable. Since ractopamine has teratogenic effects, this has been mentioned in the report, but it was not evaluated based on the international principles of extrapolating animal teratogenic effects to humans, and the teratogenic risk that Taiwan would bear after the release and the feasibility of risk management were not calculated. The above two points are enough to show that this is a "report" with major methodological flaws. |
<<: Hot stir-fry low-calorie detox recipes are delicious and good for weight loss
>>: New "Aphrodisiac Grass"! Mass production of chives in summer has become a new favorite
Recommend
What does BV mean?
BV has different meanings in different contexts, ...
Prevention of cervical precancerous lesions
Should we prevent and treat cervical precancerous...
Introduction to the three most common types of dysmenorrhea
Dysmenorrhea is a gynecological disease with many...
Detailed explanation of common symptoms of uterine fibroids
Uterine fibroids are the most common benign tumor...
What are the causes of uterine fibroids?
Among the many gynecological diseases, uterine fi...
Eat 2 light meals before meals to reduce appetite and eat less
For people who want to lose weight, it is very im...
What are the common symptoms of cervicitis?
Cervicitis can be clinically divided into acute a...
What vegetables are good for uterine fibroids?
Uterine fibroids are one of the common gynecologi...
Belly fat is the hardest to lose? 5 tips to reduce your belly fat and reshape your waist
Looking at a big belly sticking out of your waist...
Will girls have toothache when their period is coming? Yes, it can cause
Women's menstruation can cause tooth acid. Be...
How to prevent chocolate cysts?
In life, everyone should pay attention to chocola...
What are the common treatments for adnexitis?
Adnexitis is one of the diseases of women, and ma...
How to eat Mid-Autumn Festival delicacies? Four principles to get rid of the burden
The Mid-Autumn Festival is approaching, and moonc...
Diet and weight loss during menstruation can cause irregular menstruation
According to statistics, young girls make up the ...
Causes of bacterial vaginosis
There are many types of bacterial vaginosis, and ...